Please confirm that you are a healthcare professional
Omniplasma
Omniplasma is a pooled, SD treated and prion reduced plasma which was added to Prothya’s range of products in 2013.
Omniplasma is made from the plasma of voluntary and unpaid Dutch donors. The plasma is collected by Blood Bank and converted by the producer into Omniplasma (diagram). One batch of Omniplasma consists of approximately 600 -1200 donations. As Omniplasma is a pooled product, it has a constant composition of coagulation factors and other plasma proteins.

Product information
-
What is Omniplasma?
Omniplasma is a pooled, SD treated and prion-reduced plasma.
-
Why was Omniplasma being added to Prothya's range of products?
Omniplasma has been added to the product assortment on the advice of the Medical Advisory Board (MAR). Important considerations in this respect included the reduction of the risk of transmission of blood transmissible agents such as viruses and prions, and the further reduction of allergic reactions and the number of TRALI cases.
In this way, Prothya is satisfying the wishes of its customers. On the basis of the MAR’s considerations, Prothya decided to introduce Omniplasma as the preferred plasma for transfusions. In addition, the Q-plasma will remain available for certain symptoms.
-
What are the differences between the current Q-plasma and Omniplasma?
A table has been drawn up to illustrate the differences between the two.
-
What can I use Omniplasma for?
Omniplasma is a pooled plasma, the indications are the same as those for Q-plasma. Omniplasma is a medicine and detailed information in respect of the indications, contraindications, warnings, storage and shelf-life are included in the specification of the product characteristics. When only a small volume is required, for example in the case of young children, you can also make use of the split Q-plasma units. Q-plasma is used for exchange transfusions.
-
Why is Omniplasma a medicine?
Omniplasma is a pooled product and has also undergone an industrial treatment process. On that basis, it qualifies as a medicine.
-
Is Omniplasma the same as the previously supplied ESDEP?
ESDEP was supplied in the period 1996-2002. This was a pooled SD-treated plasma. There was no prion reduction step in this process. At the time, the absence of a prion reduction step was the reason given for stopping the distribution of this product.
-
What is the difference between Octaplas, Octaplas LG and Omniplasma?
Octaplas is a pooled SD-treated plasma, the plasma comes from paid and unpaid donors who live abroad.
Octaplas LG is a pooled SD-treated plasma that has undergone a prion reduction step, the plasma comes from paid and unpaid donors who live abroad.
Omniplasma undergoes the same production process as Octaplas LG, except that in its case the plasma comes from voluntary and unpaid donors living in the Netherlands.
During the production process of Octaplas LG and Omniplasma, the duration of the SD-treatment is shorter, this has a favourable effect on the protein S and alpha2-antiplasmin which are labile to SD chemicals. The shortened SD-treatment does not lead to any reduction in viral safety.
-
Can I still order Q-plasma?
There is only a limited quantity of quarantine plasma available for specific applications, for example paediatric administration, exchange transfusions for neonates and lgA-deficient patients.
-
What additional measures are being taken to prevent viral transmissions?
Each donor is screened, just as is applicable for the Q plasma supplied by Sanquin Blood Bank. In addition, NAT-tests are carried out for hepatitis A and Parvovirus B19. In the case of Parvovirus B19, the NAT-test does not have to be negative, but a value has been set for the permitted content of Parvovirus B19 DNA. Prior to production, the production pool is tested for Parvovirus B19, ultimately these pools may contain no more than 10 IU/µl Parvovirus B19 DNA.
An SD treatment is applied during the Omniplasma preparation process. This treatment is effective and makes the lipid enveloped viruses, for example HIV, HCV, HBV, inactive (West Nile virus also belongs to these types of viruses).
Neutralised antibodies against HAV and Parvovirus B19 are also present as pooled products.
-
What additional measures are being taken to prevent prion transmission?
The same exclusion criteria apply to Omniplasma as those for donors when it relates to a stay in the United Kingdom, use of growth hormones, cornea and cerebral membrane transplants, family members with Creutzfeld-Jacob disease. Additionally, in the Omniplasma preparation process, an affinity chromatography step is performed with a prion-specific ligand.
-
What additional measures are being taken in respect of bacteria and parasites?
The policy regarding travel to countries where there is malaria remains the same for Omniplasma. In addition, a sterile filtration (0.45 µm and 0.2 µm) is used as filling in the Omniplasma preparation process.
-
Can Omniplasma be delivered to the clinical chemical laboratory?
Yes, that is possible. Prothya has focused its way of working on supplying clinical chemical laboratories. To this end, an agreement between a hospital pharmacist and a clinical chemical laboratory is required. The hospital can draw this up itself or make use of an existing format. The text in this format cannot be changed. As Omniplasma is a medicine, the hospital pharmacist remains responsible.
-
Why is Omniplasma supplied per 10 units?
A frozen product is fragile. By carefully packaging and delivering Omniplasma in an outer box (containing 10 units), the chance of breakage is reduced.
-
What are the dimensions of the outer box and individual packages?
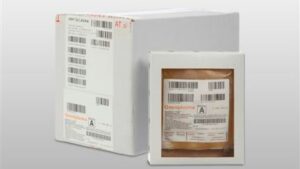
Outer box:
26 x 15.5 x 20 cm
Single package:
15 x 13 x 3 cm
-
The Omniplasma labels differ from the labels for short-life blood products, does this have consequences?
The labelling of the Omniplasma units meets the requirements of the Medicines Act (Geneesmiddelenwet) and (in respect of bar coding) meets the requirements of ISBT 128.
Compared to the structure of the labels that Sanquin Blood Bank uses for short-life blood products, there are therefore a few differences, both in the layout and in the content of the bar codes. It is important that the software is designed for this, both for your laboratory system and product scanners.
Coding of the units
Below is an example of the label for a unit of fresh, frozen (quarantine) plasma.
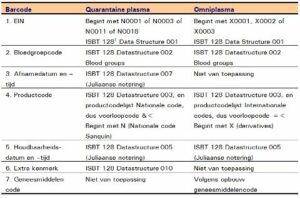
This label has six (6) bar codes, namely (from left to right and from top to bottom):
- The EIN (donation number);
- The blood group code;
- The collection date;
- The product code;
- The expiry date;
- The extra feature.

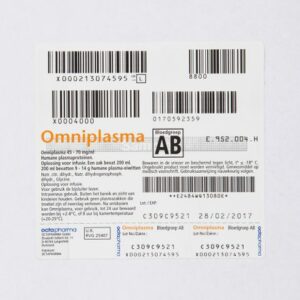
Below is an example of an Omniplasma label. This label has five (5) bar codes. The content is, from left to right and from top to bottom: (numbering in accordance with the Quarantine plasma label).
- EIN (unit number)
- The blood group code; this is encrypted: (blood group ABO) (Rhesus unknown) because Omniplasma has no RhD
- The product code
- The expiry date
- The medicine code
The differences and similarities are as follows:
Coding of the boxes
Prothya supplies Omniplasma to hospitals in outer boxes of 10 pieces. Each box has a box label that states:
- The EINs of the 10 units in the box
- The box number
- There is a label on the box which corresponds to the units in the box; this displays the data of the batch in an ISBT 128 bar code (blood group, batch number, expiry date).
This data is provided on the box for convenience and traceability, the information about the units in the box can be scanned and read without the box having to be opened. The units in the box can be quickly added to the stock, and the box as a whole can be kept in stock.
-
What is the DBC code for Omniplasma?
Since 1 January 2014, the healthcare validity code (zorgactiviteitcode) 190470 has been valid specifically for Omniplasma. Until that time, it was best to use 190404, so that it was clear that it related to plasma (that was not 190489, which was the residual group).
Contact details
The Unit of Transfusion Medicine (UTG) is part of Sanquin Blood Bank and provides advice to doctors, pharmacists, medical specialists and clinical chemists. Professionals can consult doctors from the UTG by telephone 24 hours a day, 7 days a week.
If you have any questions or comments about the labelling, please contact info@prothya.com.
-
Under what conditions should Omniplasma be stored?
Omniplasma has a shelf-life of four (4) years after being produced.
How long can Omniplasma be stored once it is thawed?
The SPC stipulates that after thawing, Omniplasma can be stored for five (5) days at +2 to +8°C and eight (8) hours at room temperature (+ 20 tot + 25°C). Medicines must be used in accordance with the SPC.
Recently, a number of articles have been published about the thawed status of Octaplas LG (same process, different plasma source) and the effect on the coagulation factors.
Keller et al. Transfusion and Apheresis Science 46 (2012) 129–136 [link]
Keller et al. Blood Transfus 2012; 10: 360-7 [link]
A. Neisser-Svae et al. Transfusion 2016; 56; 404-409 [link]
-
Where can I order Omniplasma?
Omniplasma can be ordered from Sanquin Blood Bank, where the short-life blood products are also ordered. The product can be delivered to a clinical chemical laboratory, provided there is an agreement between the hospital pharmacy and the clinical chemical laboratory.
-
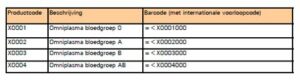
What are the product codes of Omniplasma?
In contrast to the short-life blood products, Omniplasma has an individual product code per blood group.
The table below provides an overview of the codes and descriptions:
Z-index nummers Omniplasma
15835111 omniplasma infvlst bloedgroep 0 bag 200ml
15835073 omniplasma infvlst bloedgroep a bag 200ml
15835081 omniplasma infvlst bloedgroep a+b bag 200ml
15835103 omniplasma infvlst bloedgroep b bag 200ml
-
Can Omniplasma be delivered in emergency situations?
Urgent delivery is possible. The smallest quantity that can be ordered is a box of ten pieces. Due to the longer shelf-life of Omniplasma, the risk that a product must be destroyed is low.
-
What does Omniplasma cost?
The price of Omniplasma is stipulated in the Prothya price list. Because Omniplasma is a medicine, VAT must be levied. The low rate of VAT is applicable for medicines.
-
How is Omniplasma invoiced?
Omniplasma deliveries are invoiced once a month. You will receive a separate invoice for Omniplasma, even if the delivery takes place simultaneously with other products (erythrocytes, or plasma products for example).
-
I have a question about using Omniplasma, where can I direct my question?
The Transfusion Medicine Unit (UTG) is part of Sanquin Blood Bank and provides medical advice to doctors, pharmacists, medical specialists and clinical chemists. Professionals can consult doctors from the UTG by telephone 24 hours a day, 7 days a week.
-
I want to report a side-effect of Omniplasma
Omniplasma is classified by law as a drug, therefore, it has to be reported to Prothya or Lareb.
-
What are the contraindications of Omniplasma?
These are listed in the SPC:
- IgA deficiency with documented antibodies against IgA.
- Hypersensitivity to the active ingredient, and to any of the excipients or residues from the manufacturing process.
- Serious protein S- deficiency.
In a number of these situations, it is possible to switch to Q-plasma. In case of IgA deficiency, Sanquin has special plasma available from IgA-deficient donors.
-
Why is serious protein S deficiency a contraindication?
Just as the plasmin inhibitor alpha2-antiplasmin, Protein S is labile for the SD chemicals used. Therefore, the SD treatment has an effect on the concentration of these proteins. By adapting the duration of the SD treatment in the Omniplasma production process, the values of these two proteins have improved considerably compared to those of Octaplas in the past.
-
Can Omniplasma be used in the apheresis of a TTP patient?
Omniplasma can be used in the treatment of TTP. The indication is included in the SPC. In addition, there is literature about the composition of the product specifically for ADAMTS13 concentration and activity as well as factor H for the treatment of atypical HUS.
Edel et al. Efficacy and Safety Profile of Solvent/Detergent Plasma in the Treatment of Acute Thrombotic Thrombocytopenic Purpura: A Single-Center Experience. Transfus Med Hemother 2010; 37:13–19
Heger et al. Normal levels of ADAMTS13 and factor H are present in the pharmaceutically licensed plasma for transfusion (Octaplas®) and in the universally applicable plasma (Uniplas) in development. Vox Sanguinis (2007) 92, 206–212
-
To what extent should the volume difference between Q-plasma and Omniplasma be taken into account?
A unit of Omniplasma contains 200 ml and Q-plasma contains 310 ml. Experience in Finland shows that a 15% increase was witnessed in a number of units. That would mean that generally the number of units suffices. Where dosing is by ml, as with TTP for example, more units will be needed.
-
What are the benefits of a pooled product?
Pooling results in a product with a standardised composition, which makes the clinical effect more predictable. In addition, there are also advantages with regard to transfusion reactions:
- TRALI: by pooling both male and female plasma, any HLA and HNA antibodies are diluted and/or neutralised. Hemovigilance databases in other countries have not reported any
- TRALIs when using Octaplas.
- Allergic reactions: allergens are also diluted in the pool. Based on the Austrian hemovigilance database, a reduction of 15.6% has been seen in allergic reactions. N.B. reactions due to anti-IgA can also occur with this product, use should then be made of IgA-deficient Q-plasma.
-
Omniplasma documents
An overview of relevant documents for Omniplasma (in Dutch):
- Praktische aspecten bij het ontdooien van Omniplasma
- Overeenkomst tussen ziekenhuisapotheker en klinisch chemisch laboratorium
- Toelichting ZAPO-verklaring
- Begeleidend schrijven Omniplasma 16 juli 2014
- Checklist voor implementatie van Omniplasma
- Etikettering Omniplasma
- Traceability en origine van donaties voor Omniplasa
In addition to the general description from the SPC, Prothya provides a number of SOPs that describe the thawing of Omniplasma using different equipment:
Q&A serialization Omniplasma
-
What exactly is the Falsified Medicines Directive?
The Falsified Medicines Directive (FMD; 2016/161/EC) became effective on 9 February 2019. This European regulation is intended to prevent falsified medicines entering the legal distribution chain (and, therefore, reaching patients). -
Does the Falsified Medicines Directive also apply to Omniplasma?
The FMD applies to medicines which are only available on prescription and therefore also applies to Omniplasma. “Advanced therapy medical products which consist or are composed of tissue or cell material,’’ are not considered to be subject to the FMD in all pharmaceutical forms and concentrations. Is it possible to include Omniplasma in this category? Omniplasma does not fall under the category “Advanced therapy medical products (ATMP) which consist or are composed of tissue or cell material’’. Examples of ATMPs are: products for tissue manipulation, gene and somatic cell therapy. Omniplasma is a medicine which has plasma as its raw material. A large number of donor plasma units are pooled whereby cells and cell debris are removed. Subsequently, the pharmaceutical production process consists of Solvent Detergent treatment, removal of these chemicals and a prion reduction step. All this qualifies Omniplasma as a medicine. In this sense, it is, for example, comparable to IVIg and prothrombin complex concentrate. Medicines made from plasma are subject to the FMD. -
Which safety features are added to the medicine?
1) A unique identification feature, in the form of a 2D matrix, on the basis of which the authenticity and identity of the individual packaging of a medicine can be established. 2) A seal. -
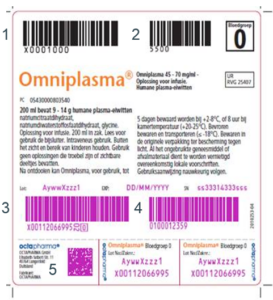
Where is the 2D-matrix code placed?
The 2D matrix is placed on a removable flag label which is, in turn, placed on the Omniplasma product label. See the figure below, this shows:- the product code
- the blood group
- the unit identification number
- the shelf-life
- the 2D matrix on the removable flag label
-
What does the label with 2D matrix code look like and which elements are processed in the 2D matrix code?
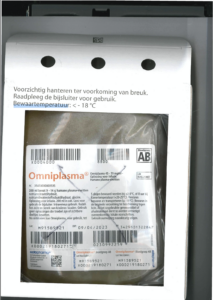 When reading out the 2D matrix code, the following features are cited:
(01) the Global Trade Identification Number, GTIN
(21) the Serial number
(10) the Batch number
(17) the Expiry date
When reading out the 2D matrix code, the following features are cited:
(01) the Global Trade Identification Number, GTIN
(21) the Serial number
(10) the Batch number
(17) the Expiry date -
How was the position of the 2D matrix code determined?
The manufacturer and the registration holder, Octapharma, submitted two proposals. These two proposals took account of: (i) the FMD and (ii) the technical feasibility. These two proposals were submitted to the working party Technology and Logistics (Techniek en Logistiek) of the National Users Council (Landelijke Gebruikersraad) and to the Dutch Hospital Pharmacists' Association (Nederlandse Vereniging van Ziekenhuisapothekers, NVZA). On the basis of these proposals, a decision was taken to place the 2D-matrix code on a removable flag label. The decisive factor was the flexibility the removable flag label offers. -
What information is shown on the 2D matrix code?
The unique identifier contains the product code, serial number, batch number and the expiry date. The producer registers the serial numbers in a central database. Each serial number must be checked and deregistered from the database before being delivered to a patient. -
The new label does not have a separate code with the ZI-number, can this be found in another way?
The 2D matrix code includes the GTIN (Global Trade Identification Number) associated with the product. In the pharmacists’ system (G-standard), a link has been made between the GTIN and the ZI-number. -
Is the unit identification number not sufficient?
The EIN code is not sufficient, this does not include the elements required for the FMD, nor is it registered in the database. Moreover, this relates to another code (linear code, ISBT128). -
Does Omniplasma also have a seal?
Each unit of Omniplasma is wrapped in sealed foil. This measure is sufficient. -
How does implementing the FMD in respect of Omniplasma affect the customers?
- There is an additional action: deregistering the unit from the European database. This requires an ICT solution.
- The lay-out of the label has been changed and the linear codes corresponding to the EIN and the expiry date are no longer in the usual position. This could impact the scan order.
- It may be necessary to adjust the internal SOPs and to review the existing agreements with pharmacists and hospital pharmacists.
-
When should Omniplasma be deregistered?
The law states that the product should be deregistered when it is handed over (delivered to the patient or his/her representative). In practice, many pharmacists prefer deregistering it when they add it to their stock. In respect of Omniplasma, account should be taken of the following:- The 2D matrix is attached to a part of the primary bag’s label. On delivery that bag is frozen and (therefore) not slippery. In addition, the primary bag is sealed in foil.
- Only after thawing are the bag and primary label released. For the purposes of deregistration, the part that contains the 2D matrix is removable.
-
Can the flag label be detached easily?
After being thawed, the removable labels are easy to detach. And, at that moment, part of the label can be removed, you could try that beforehand. -
How long can the units without a 2D-matrix code still be used?
Omniplasma which was produced and released before 9 February 2019, i.e. before the FMD came into effect, is subject to a transitional arrangement. These products neither have to be nor can be deregistered in the central database. As long as the shelf-life permits, a product can be used. -
Who is responsible for deregistering Omniplasma?
Omniplasma is a medicine. As is the case for all medicines, the pharmacist or hospital pharmacist is responsible for complying with all the required actions. Actions may be delegated, but not the responsibility. -
Can Sanquin not deregister Omniplasma itself?
Although Sanquin acts as a wholesaler, there is no opportunity for Sanquin to deregister the product prior to delivery. The regulation does not allow this. -
Is it possible to deregister via the LIMS?
There are various ICT solutions which hospitals have chosen. LIMS is not, as a standard, equipped to deregister medicine. The responsibility for the deregistration lies with the pharmacist or hospital pharmacist. It is advisable to agree with the pharmacist, or hospital pharmacist, what the most suitable institution is locally and whether the LIMS is one of the options. If this is an option, the possibilities should be examined with the supplier of the LIMS. -
What happens if the Omniplasma is not deregistered?
Medicines are not intended to be administered if their identity and authenticity have not been checked. The FMD is a European regulation that prescribes that certain actions must be carried out. Units which are not deregistered remain in the database. Eventually, medicines whose shelf-life has expired will be cleared out. -
What happens if the Omniplasma is - accidentally - deregistered twice?
Deregistration will cause an error message in the European database. -
Should a unit of Omniplasma which cannot be administered (for example due to damage, leakage, flakes) also be deregistered?
All units have to be deregistered.
